A 40 cent plasma chamber
that demonstrates quantum mechanics
Yes, it’s a simple neon lamp, but it’s also a plasma chamber that demonstrates a fascinating and important
difference between classical mechanics and quantum mechanics, or quantum physics. An incandescent lamp filament
emits light that smoothly spans the entire range of the visible spectrum. By comparison, the ionized plasma within
a neon lamp emits only very specific wavelengths of light with little or no emission in between those lines. The
explanation for that came from a physicist in 1913 named Niels Bohr.

Physicist Niels Bohr
Other scientists had been noticing these emission lines when they heated certain elements to the point where they
gave off light. It was a mystery why these spectral lines appeared the way they did instead of a smoother spectrum
like they had seen from the Sun. To explain this Niels Bohr theorized a model of the atom in which electron
particles orbited around the atom’s nucleus. In his model there was a certain number of electrons for each of the
different elements, and these electrons had orbits of very specific energy levels.
When the element was heated, or electrically excited, the electrons jumped to a higher orbital energy level then
fell back to their original orbital level, giving off photons of light as a way to release the difference in
energy. The wavelength, or color, of the photons was specific to the exact change in electron energy levels. In
order for the emission lines to be consistent – which they are – it was necessary for these electron orbital
energy levels to be exact quantities (quanta) with no energy values in between. This jump between orbital energy
levels was called the quantum leap and is where the term originated.
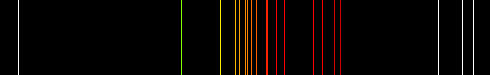
(Spectral emission lines of neon plasma)
The specific, or quantum energy levels within the atom that the electrons orbit at are very different from the
energy levels in classical physics - the physical world we normally observe. In classical physics energy can have
any value. A rotary dimmer switch, for example, can be infinitely variable between fully-off and fully-on, but
quantum energy levels must be precise amounts with no variation in between.
It’s still a scientific mystery why quantum physics and classical physics are different in this way. The Bohr
model of the atom is still theoretical, since we can’t directly observe that small of a scale to see the
electrons actually orbiting, but the model still holds up as an explanation against all physics experiments so
far.
Notes:
Quantum physics and classical physics are interchangeable terms with quantum mechanics and classical mechanics.
Images used under GNU license
Links:
Niels Bohr
Bohr atomic model
neon lamp

